Interaction of Methanol and Water with an Zeolite Acid Site
There is a movie at the end of this article!
 Zeolites are important catalysts used in petrochemistry and the
chemical industry for the production of fine chemicals. Zeolites
are silicon-dioxide modifications like glass or quartz
sand. However, in contrast to glass or quartz, the r crystal
structure of zeolites exhibits large pores and cages. The pores
are sufficiently large that molecules with specific shapes can
pass right into the interior of the crystal. If the "internal
surface", namely that of the cages, is covered with reactive
centers, zeolites are catalytically active and speed up and
direct the reactions to the desired reaction product.
Zeolites are important catalysts used in petrochemistry and the
chemical industry for the production of fine chemicals. Zeolites
are silicon-dioxide modifications like glass or quartz
sand. However, in contrast to glass or quartz, the r crystal
structure of zeolites exhibits large pores and cages. The pores
are sufficiently large that molecules with specific shapes can
pass right into the interior of the crystal. If the "internal
surface", namely that of the cages, is covered with reactive
centers, zeolites are catalytically active and speed up and
direct the reactions to the desired reaction product.
A detailed understanding of the reactions occuring inside the zeolite is important to develop new catalysts and catalytic processes. However, the reaction process is hidden in the interior of the material, prohibiting experimental access by, for example, surface techniques that are well developed. With the help of modern simulation techniques such as density functional theory and ab-initio molecular dynamics, we can explore in detail what is hidden otherwise. Our model assumptions are verified by comparing calculated data with experiment. Once we are sure that we focus on the correct process, we can deduce a wealth of information and watch the process as they occur.
Despite its enormous scientific and technological importance, one of the fundamental processes in zeolites, namely the interaction of polar molecules with acid sites has remained elusive. These processes are key to the understanding of the well known Methanol-to-Gasoline process, which is a major contender for the use of recyclable energy resources. The interpretation of the experimental results has been under long dispute. This is the reason for exploring these processes in our simulations.
Our simulations show that a loosely bound proton of the so-called acid center is not transfered to the polar molecules at low coverages. The calculated vibrations have been in good aggrement with experimental infrared spectra confirming the validity of the results. However, it was unclear how these protons can participate in reactions while the remain at the lattice. The origin of this apparent contradiction could be resolved by increasing the coverage of the polar molecules. Already two polar molecules at an acid site proton are able to remove the proton from the lattice and bind it in a way similar to a proton in liquid methanol or water. Thus the reactivity can be explained similarly to that of acid solutions, with the difference of a huge number of available protons and the steric contstraints of the cages.
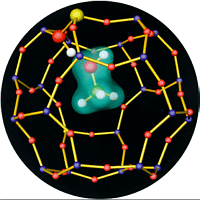
Watch here a movie of the methanol molecule and three water molecules in a sodalite cage. (Please be patient in the beginning. You will need the Realplayer to view it) The first movie shows a methanol molecule intreacting with a zeolite cage. The zeolite cage has been cut open for better visibility. The yellow ball is an aluminum atom in the place of a silicon atom of the zeolite framework. It is responsible for a proton to attach at an oxygen atom (red balls) next to it. The molecule forms weak, socalled hydrogen bonds to the lattice. One is very strong an remains intact during almost the entire simulation.
The second part of the movie shows three water molecues in the cage as an example for larger coverages. The proton has been attached to the lattice initially but the soon passes over to the water "droplet", creating an extremely acid solution.
Sources:
E. Nusterer, P.E. Blöchl and K. Schwarz,
Angew.Chem. Int'l Ed. Engl, 35, 175 (1996)
For more information see
- "Structure and Dynamics of Methanol in a Zeolite",
E. Nusterer, P.E. Blöchl and K. Schwarz, Angew.Chem. Int'l Ed. Engl, 35, 175 (1996) - "Interaction of Water and Methanol with a Zeolite at High Coverages",
E. Nusterer, P.E. Blöchl and K. Schwarz, Chem. Phys. Lett. 253, 448 (1996).